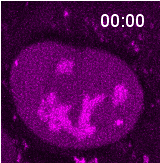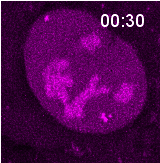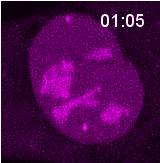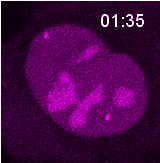
Research @ the Noordermeer Lab
DNA double-strand breaks (DSBs) are one of the most cytotoxic lesions and can lead to diseases such as cancer when repaired erroneously or left unrepaired. Our cells are equipped with several mechanisms to repair these DSBs, showing differences in efficiency and fidelity. Using a variety of systemic approaches (large scale CRISPR and yeast-two-hybrid screens, proteomics) and dedicated molecular biology approaches to assess genomic stability and break repair we aim to understand how the cell regulates its DSB repair and how the repair mechanisms communicate with other cellular processes such as replication.
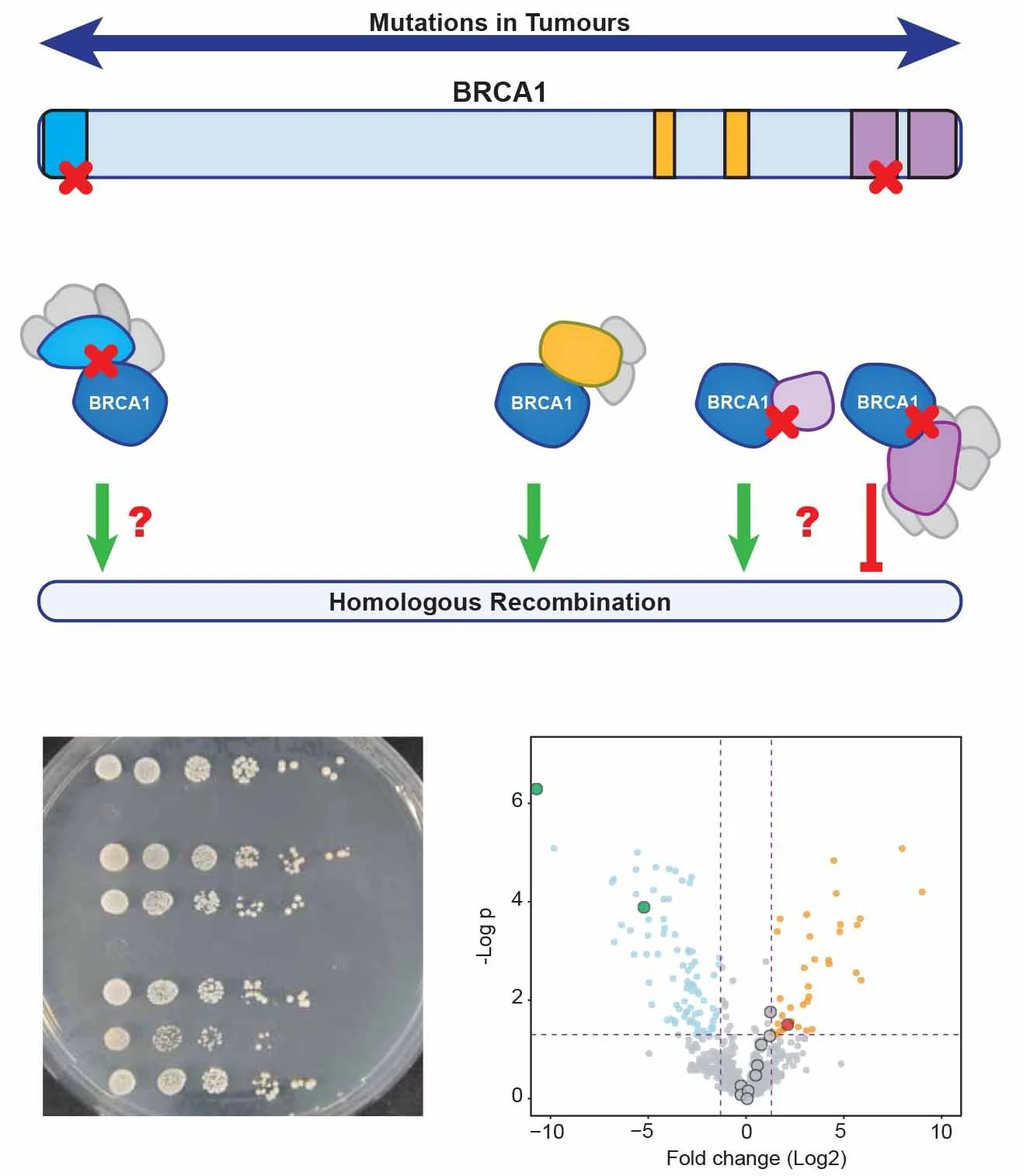
BRCA1 complex formation
BRCA1 is a key protein for the repair of DSBs via Homologous Recombination (HR). BRCA1 mutations occur in a wide variety of tumours, including hereditary forms of breast and ovarian cancer. BRCA1-mutated tumours are characterised by gross chromosomal instability. Next to many truncating mutations, leading to a fully dysfunctional BRCA1, many missense mutations and also variants of unknown significance have been identified in tumours. However, how all these different mutations in BRCA1 are involved in cancer development and therapy response is often still unclear.
One of the main complexities in studying BRCA1 function is the fact that it forms several multi-protein complexes via its different protein domains, most of them stimulating HR, but others – counterintuitively – inhibiting HR. In our lab we aim to better understand how all these different BRCA1-complexes cooperate to maintain genomic stability and how mutations disrupting single complexes affect tumorigenesis.
We correlate the functional studies on complex-disrupting BRCA1 mutations to clinical data of cancer patients with such mutations to improve risk stratification of patients with BRCA1-mutated tumours. Furthermore, we study the genetic relationship between BRCA1 and its different complex members via a collection of genome-wide CRISPR/Cas9 synthetic lethality screens. This will enhance our understanding of the unique and common functions of these complexes in cellular homeostasis. With the data of the screens, we are identifying novel druggable pathways and targets to treat cancer patients with specific complex-disrupting mutations.
Identifying novel vulnerabilities of homologous recombination deficient tumours
DSB repair pathway choice
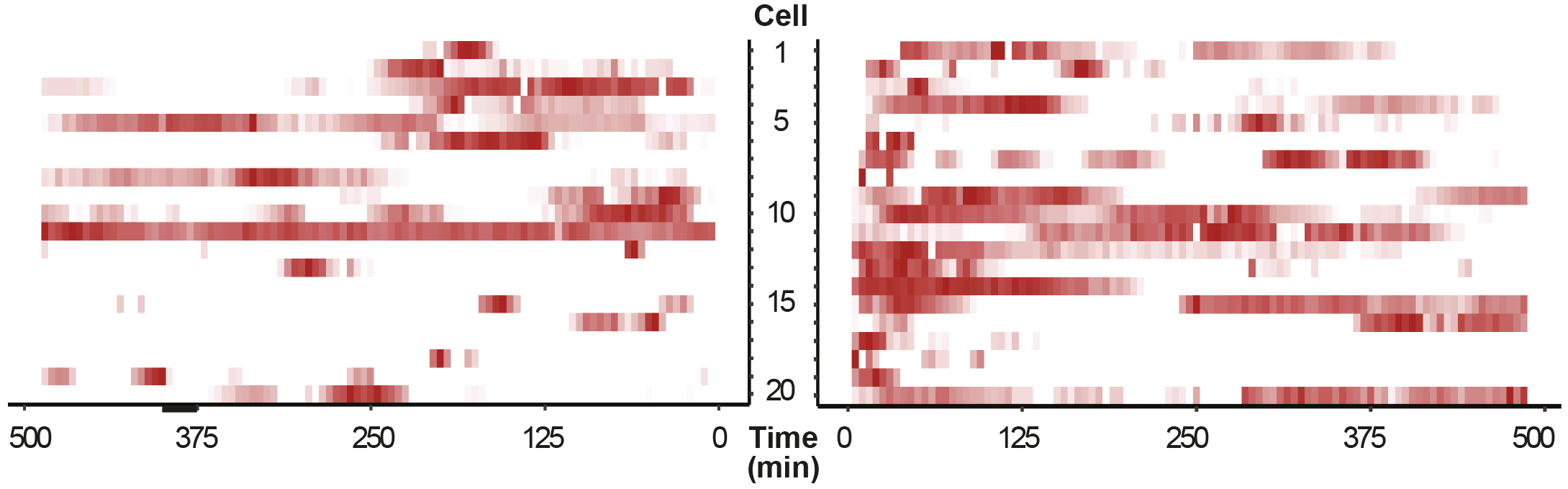
The cell is equipped with multiple repair pathways to resolve breaks. These pathways vary in their fidelity for repair. When a break occurs, the cell needs to make a choice which pathway to activate. Although we already know that many cellular aspects, such as cell cycle stage, chromatin context and break aetiology, affect pathway choice, we still do not fully understand how a cell makes this decision. Our lab investigates this pathway choice by performing high resolution live cell imaging experiments to better understand and predict the outcome of repair of breaks with different origins. Furthermore, we are developing novel sequencing techniques to read-out in real-time what repair mechanism was used by the cell upon induction of a specific break.